
Role of Serum Adenosine Deaminase (ADA) in the Diagnosis of Tuberculosis and Other Respiratory Diseases
Authored by - Arun Krishnarao Tadas, Komal Waman Meshram, Sanjay B Agarwal, Poonam Lalla
Published in - International Journal of Clinical Biochemistry and Research 2023 October, (3):242–246
DOI- https://doi.org/10.18231/j.ijcbr.2023.042
ABSTRACT
Background & Objectives: The WHO Tuberculosis (TB) statistics for India in 2021 gave an estimated incidence figure of 25,90,000 cases, i.e., about 40% of Indian population is infected with TB. There are different investigative methods available for TB diagnosis like ZN-staining of M.tb, which lacks sensitivity & another method of Mycobacterial culture takes around 6-8 weeks to isolate M.tb in culture which results in delayed diagnosis & treatment and meanwhile further progression of disease. Other sensitive methods like PCR & CBNAAT are costly & they require skilled personnel & lots of equipment. Therefore, there is a need of simple, cost-effective, rapid & reliable test which can be easily carried out in the clinical laboratories of resource limited countries. In some previous studies, the level of ADA in effusion fluids was used for the diagnosis of TB, but it is not always possible to access effusion fluid & it requires skilled personnel. Thus, the aim of the present study is to evaluate the usefulness of measuring the serum level of ADA as noninvasive biochemical marker in early diagnosis of TB.
Materials and Methods: The present cross-sectional study was conducted on 200 serum ADA samples. Patient samples were divided into four groups based on their diagnosis, i.e., 50 patients with pulmonary TB, 50 patients with extra-pulmonary TB, 50 patients with respiratory infections other than TB & 50 healthy people not having TB.
Results: The ADA level for each group was presented as mean + SD & compared by applying post hoc Bonferroni test which showed that the pulmonary TB group was significantly different from the other 3 groups with p<0.001. According to ROC curve analysis, the best cutoff point was 21.8 IU/L at which sensitivity & specificity were 88% & 87% respectively.
Conclusion: Serum ADA activity with high sensitivity & specificity percentage can be used as a reliable marker in the diagnosis of TB & to differentiate TB from other respiratory illness.
INTRODUCTION
Tuberculosis (TB), an infectious disease caused by Mycobacterium tuberculosis (M.tb) bacteria, generally affects the lungs, but it can also affect other parts of the body. 1 The World Health Organization (WHO) TB statistics for India in year 2021 gave an estimated incidence figure of 25,90,000 cases. This is a rate of 188 per 1,00,000 population, i.e., about 40% of Indian population is infected with TB bacteria & the vast majority of whom have latent TB rather than TB disease. 2 There are different investigative methods available for diagnosis of TB. 3–6 Conventional smear microscopy with Ziehl-Neelsen (ZN) stain lacks sensitivity as it has very low detection rate around 16-50% & large portion remains negative in spite of clinical & radiological signs suggestive of TB. 7 Mycobacterial culture being gold standard method for diagnosis of TB possesses major limitation of longer incubation period that is around 6-8 weeks to get isolate in culture to which antibiotic susceptibility testing can further add 3-6 weeks. Meanwhile the disease may progress which cause delay in an appropriate treatment & increase hospitalization. 8 Recent advances in molecular techniques like polymerase chain reaction (PCR) & cartridge based nucleic acid amplification test (CB-NAAT) have shortened the time needed to diagnose TB, leading to improved case detection & management but these methods require skilled personnel & expensive instruments. This limits their usefulness & access in areas with insufficient resources. 9,10 Therefore, there is a need of simple, cost effective, rapid & reliable test which can be easily execute in the clinical laboratories of resource limited areas of developing countries like India. Some previous studies have used the level of adenosine deaminase (ADA) in effusion fluids to diagnose TB. 11 ADA (E.C. 3.5.4.4) is an enzyme of purine metabolism which catalyzes the irreversible deamination of adenosine into inosine. 12 ADA plays an important role in activation of lymphocytes. 13 Mycobacterial antigens infect macrophages which cause activation of T-cell lymphocytes resulting in increasing levels of ADA in TB. 14 ADA may be released from effusion fluids & be detected in serum of patients with active TB. As it is not always possible to access effusion fluid in pulmonary & extrapulmonary TB everywhere because it requires skilled personnel, therefore it would be helpful to take advantage of serum level of ADA to diagnose TB.
OBJECTIVE
The aim of the present study is to evaluate the relevance of measuring serum ADA activity in TB patients as a non-invasive biochemical marker in the early diagnosis of disease.
MATERIALS AND METHODS
The present cross-sectional study was completed over a period of 12 months, i.e., from May-2021 to April-2022 conducted on patients attending out-patient department & patients getting admitted in wards of pulmonary medicine department of one of tertiary health centers of central India. 200 age & sex matched randomly assigned subjects belonging to 18-50 years of age group diagnosed based on their CB-NAAT & histopathology report, were divided into four groups:
- Group I= 50 patients newly diagnosed with pulmonary TB
- Group II= 50 patients newly diagnosed with extrapulmonary TB
- Group III= 50 patients with respiratory infections other than TB
- Group IV= 50 healthy people not having TB
3.1. Exclusion criteria Patients having the following ailments
- HIV/AIDS
- Liver Diseases
- Typhoid Fever
- Infectious Mononucleosis
- Gout / Rheumatoid Arthritis
- Renal Failure
- Corticosteroid Therapy
- Anti-TB treatment
After obtaining an informed consent, about 2ml of blood was drawn by venipuncture in plain tube from each patient following all aseptic techniques & immediately sent to laboratory. Then samples were centrifuged at 2,500 rpm for 10 minutes & the serum was separated into clean, dry & sterile vials. ADA activity was measured on the same day of collection of samples on Fully Automated Biochemistry Analyzer XL-640 manufactured by Erba Transasia Bio-Medicals Ltd.
3.2. Statistical analysis
All the results were expressed as mean + Standard Deviation (SD). Mean serum ADA level for each group was assessed by analysis of variance (ANOVA) test. Receiver operating characteristic (ROC) curve was used to determine a cut-off value for the ADA test. In addition, the diagnostic usefulness of ADA was assessed in terms of sensitivity & specificity. Area under curve was used to determine the discriminatory ability of the assay to distinguish between TB (pulmonary & extrapulmonary) from non-TB respiratory infections. Post Hoc Bonferroni test was used to determine the significant differences between the groups & p-value <0.001 was considered as statistically significant. All calculations were performed by the statistical package for social sciences (SPSS) software version 15.
RESULTS
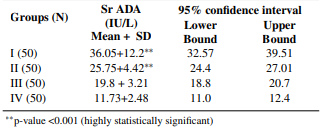
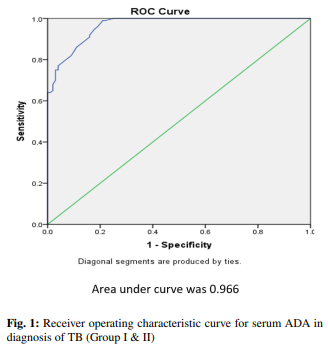
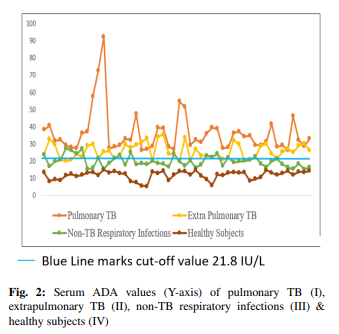
The mean levels of serum ADA were found to be significantly higher in the patients with active TB (groups I & II) in comparison to the ones who were suffering from any other respiratory illness (group III) & healthy subjects (group IV) (Table 2). Groups were compared by using post HOC Bonferroni test & test result showed highly significant difference between TB groups (group I & II) and other two groups, i.e., non-TB respiratory infections & Healthy Subjects (group III & IV) (p-value <0.001). However, no significant relationship was observed between pulmonary & extrapulmonary TB groups (p-value = 0.222). Although mean ADA level of non-TB respiratory infection group (Group III) is higher than mean ADA value of healthy subjects (Group IV), the difference is not significant (p-value = 0.509). The cutoff value of serum ADA activity for the diagnosis of TB established by the ROC curve method was 21.8 IU/L (Figure 1). 88% of patients (88 out of 100) with active pulmonary & extrapulmonary TB had serum ADA values above cutoff level. 26% of patients (13 out of 50) with other respiratory illness had serum ADA values more than cutoff level while none of the healthy subjects showed serum ADA values above the cutoff level (Figure 2). The sensitivity of serum ADA for diagnosing TB was 88% & the specificity was 87%. The calculated positive predictive value (PPV) was 87.13% & negative predictive value (NPV) was 87.88%.
Table 1: Age & gender wise distribution of pulmonary TB (I), extrapulmonary TB (II), non-TB respiratory infections (III) & healthy subjects (IV)
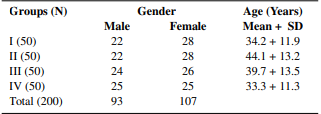
Table 2: Levels of serum ADA in pulmonary TB (I), extrapulmonary TB (II), non-TB respiratory infections (III) & healthy subjects (IV)
DISCUSSION
Despite all the huge advances in the diagnosis & treatment of TB, the disease is still a major health problem in many parts of the world, especially in developing countries. Lack of timely TB diagnosis is the major cause of failure in managing the disease. Although the standard diagnosis of TB is based on isolation of M.tb in culture or direct observation of acid fast bacilli in ZN-stained sputum smear 15 yet, other diagnostic methods with shorter duration & acceptable sensitivity & specificity are necessary. Rapid diagnostic methods of TB by molecular techniques like PCR & CBNAAT16–18 is not available in low-income areas due to the high cost of these methods. There is need to practice such tests which determine the biological markers of TB infection such as ADA levels. ADA is an enzyme in the purine salvage pathway that catalyzes the conversion of adenosine & deoxyadenosine to inosine & deoxy-inosine with the release of ammonia. 19,20 Monocyte or macrophage activation by intracellular infections & inflammatory diseases leads to the release of ADA. 21,22 Therefore, its increased concentration may be found in fluids & serum present in zones of TB infection, which can be used in diagnosis. 23 The importance of ADA activity measurement in pleural, peritoneal, synovial, cerebrospinal fluids has already been proven for the diagnosis of pulmonary & other extrapulmonary TB. 24,25 Piras MA et al. were first to report high ADA in tubercular pleural effusion. 26 However very limited studies are available for ADA levels in serum. The ease of venipuncture over access to other body fluids is going to be more helpful. Also, there is no such data available regarding any research on the diagnosis of TB using serum ADA level in central India. In our study, mean serum ADA in patients with pulmonary & extrapulmonary TB is significantly higher than other patient group with respiratory illness (p-value <0.001). The results are concurrent with Agarwal MK et al., 27 Afrasiabian S et al., 28 Dilmac A et al. 29, Salmanzadeh S et al., 30 Atta S et al. 31 and Al-sham-mary FJ. 32 This finding is in contrast with the study by Conde MB et al. 33 which shows that the mean serum ADA value among patients with active TB is similar to patients with other respiratory diseases (p-value =1.0). Our results also showed that mean serum ADA in patients with pulmonary & extrapulmonary TB is clearly higher than that of healthy subject group (p-value <0.001). This finding is consistent with studies of Agarwal MK et al., 27 Afrasiabian S et al., 28 Badade ZG et al., 34 Chander A et al., 35 and Titarenko OT et al. 36 Bolursaz MR et al. 37 believed that although serum ADA level in pulmonary TB patients is higher than in normal individuals, ADA should not be considered as a suitable marker for differentiation between pulmonary TB & other pulmonary infections. The current study showed high sensitivity (88%) & specificity (87%) of Serum ADA for diagnosing TB at cutoff value of 21.8 IU/L. From the above it is evident that estimation of serum ADA is reliable & useful test for diagnosis of TB. The area under ROC curve was 0.966 which shows that serum ADA can differentiate TB from other respiratory illnesses. A study by Agarwal MK et al. 27 shows 98% sensitivity & 100% specificity at serum ADA level of 33 IU/L. In Hassanein K et al. 38 study with an ADA level of 26.2 IU/L, sensitivity & specificity were 95% & 83.3% respectively. Another study by Afrasiabian S et al. 28 also showed high sensitivity (92.7%) & specificity (88.1%) for TB diagnosis at serum ADA cutoff value of 14 IU/L. This observation is in contrast with the study by Salmanzadeh et al., 30 Conde MB et al., 33 Farazi A et al. 39 that showed low sensitivity of serum ADA assay for differentiating TB from other respiratory illnesses & thus disapproved its utility for TB diagnosis. Such differences are may be due to disease severity, immune status, genetic differences & accuracy of doing laboratory examination. There are some limitations of our study. This study involves small number of patients of single geographical area & study was the first of its kind in the region to evaluate the adequacy of serum ADA in diagnosis of TB & other respiratory illness. Therefore, conclusion based on one study should be accepted with caution. Some other diseases of cell mediated immunity like Typhoid fever, Infectious Mononucleosis, Rheumatoid arthritis, liver diseases are expected to be associated with high serum ADA levels. 32 We have excluded these diseases based on clinical presentation & not on the basis of specific diagnostic test. Therefore, further controlled studies with larger group are recommended.
CONCLUSION
According to this study, serum ADA level with high sensitivity & specificity may be proposed as reliable & useful index for diagnosis of TB, but the findings should be correlated with clinical presentation of disease. Serum ADA can also be used to differentiate TB from other respiratory illnesses. Thus, it can be included in routine diagnosis & prognosis of tuberculosis. However, further validation with larger studies must be done.
SOURCE OF FUNDING: None
CONFLICT OF INTEREST: None
REFERENCES
- Stevanovic G, Pelemis M, Pavlovic M, Lavadinovic L, Dakic Z, Milosevic I, et al. Significance of adenosine deaminase serum concentrations in the diagnosis of extra-pulmonary tuberculosis. J IMAB. 2011;17:130–4.
- TB Statistics India. Available from: https://tbfacts.org/tb-statistics-india/.
- Steingart KR, Ng V, Henry M, Hopewell PC, Ramsay A, Cunningham J, et al. Sputum processing methods to improve the sensitivity of smear microscopy for tuberculosis: A systematic review. Lancet Infect Dis. 2006;10(10):664–74.
- Perkins MD, Roscigno G, Zumla A. Progress towards improved tuberculosis diagnostics for developing countries. Lancet. 2006;367(9514):942–3.
- Storla DG, Yimer S, Bjune GA. A systematic review of delay in the diagnosis and treatment of tuberculosis. BMC Public Health. 2008;8:15. doi:10.1186/1471-2458-8-15.
- Diagnostic and treatment delay in Tuberculosis. World Health Organization; 2006. Available from: https://applications.emro.who. int/dsaf/dsa710.pdf.
- Dimakou K, Hillas G, Bakakos P. Adenosine deaminase activity and its isoenzymes in the sputum of patients with pulmonary tuberculosis. Int J Tuberc Lung Dis. 2009;13(6):744–8.
- Lamsal M, Gautam N, Bhatta N, Majhi S, Baral N, Bhattacharya SK. Diagnostic utility of adenosine deaminase (ADA) activity in pleural fluid and serum of tuberculous and non-tuberculous respiratory disease patients. Southeast Asian J Trop Med Public Health. 2007;38(2):363– 9.
- Wallis RS, Pai M, Dick M, Doherty TM, Walzl G, Perkins MD. Biomarkers and diagnostics for tuberculosis: progress, needs, and translation into practice. Lancet. 2010;375(9729):1920–37.
- Jobayer M, Shamsuzzaman SM, Mamun KZ. Rapid Diagnosis of Pulmonary Tuberculosis from Sputum by Polymerase Chain Reaction. Arch Clin Infect Dis. 2014;9(2):20694.
- Greco S, Girardi E, Masciangelo R, Capoccetta GB, Saltini C. Adenosine Deaminase and interferon gamma measurements for the diagnosis of tuberculous pleurisy: a metaanalysis. Int J Tuberc Lung Dis. 2003;7(8):777–86.
- Barua R, Hossain M. Adenosine Deaminase in Diagnosis of Tuberculosis: A Review. Anwer Khan Mod Med Coll J. 2014;5(2):43– 8.
- Russo M, Giancane R, Apice G, Galanti B. Adenosine deaminase and purine nucleoside phosphorylase activities in peripheral lymphocytes from patients with solid tumours. Br J Cancer. 1981;43(2):196–200.
- Collazos J, Espan4a P, Mayo J, Martinez E, Izquierdo F. Sequential evaluation of serum adenosine deaminase in patients treated for tuberculosis. Chest. 1998;114(2):432–5.
- Alavi SM, Khoshkhoy MM. Pulmonary tuberculosis and diabetes mellitus: Co-existence of both diseases in patients admitted in a teaching hospital in the southwest of Iran. Caspian J Intern Med. 2012;3(2):421–4.
- Lassence AD, Lecossier D, Pierre C, Cadranel J, Stern M, Hance AJ. Detection of mycobacterial DNA in pleural fluid from patients with tuberculous pleurisy by means of the polymerase chain reaction: comparison of two protocols. Thorax. 1992;47(4):265–9.
- Querol JM, Minguez J, Garcia-Sanchez E, Farga MA, Gimenco C, Garcia-De-Lomas J. Rapid diagnosis of pleural tuberculosis by polymerase chain reaction. Crit Care Med. 1995;152:1977–81.
- Villena V, Rebollo MJ, Aguado JM, Galan A, Lopez-Encuentra A, Palenque E. Polymerase chain reaction for the diagnosis of pleural tuberculosis in immunocompromised and immunocompetent patients. Clin Infect Dis. 1998;26(1):212–4.
- Jhamaria JP, Jenaw RK, Luh SK, Mathur DK, Parihar HL, Sharma SK. Serum adenosine deaminase (ADA) in differential diagnosis of pulmonary tuberculosis and common non tubercular respiratory diseases. Ind J Tub. 1988;35:25–7.
- Zamalloa A, Gomez JT. Diagnostic accuracy of adenosine deaminase and lymphocyte proportion in pleural fluid for tuberculous pleurisy in different prevalence scenarios. PLoS One. 2012;7:e38729.
- Gupta BK, Bharat V, Bandyopadhyay D. Sensitivity, specificity, negative and positive predictive values of adenosine deaminase in patients of tubercular and nontubercular serosal effusion in India. J Clin Med Res. 2010;2(3):121–6.
- Sharma SK, Suresh V, Mohan A, Kaur P, Saha P, Kumar A, et al. A prospective study of sensitivity and specificity of adenosine deaminase estimation in the diagnosis of tuberculosis pleural effusion. Indian J Chest Dis Allied Sci. 2001;43(3):149–55.
- Mathur PC, Tiwari KK, Sushima T, Tiwari D. Diagnostic value of adenosine deaminase (ADA) activity in tubercular serositis. Indian J Tuberc. 2006;53:92–5.
- Saleh MA, Hammad E, Ramadan MM, El-Rahman AA, and AFE. Use of adenosine deaminase measurements and QuantiFERON in the rapid diagnosis of tuberculous peritonitis. J Med Microbiol. 2012;61(Pt 4):514–9.
- Tuon FF, Silva VI, Almeida GM, Antonangelo L, Ho YL. The usefulness of adenosine deaminase in the diagnosis of tuberculous pericarditis. Rev Inst Med Trop Sao Paulo. 2007;49:165–70.
- Piras MA, Gakis C, Budroni M, Andreoni G. Adenosine deaminase activity in pleural effusions: An aid to differential diagnosis. Br Med J. 1978;2(6154):1751–2.
- Agarwal MKN, Mukerji PK, Srivastava VML. A study of serum adenosine deaminase activity in sputum negative patients of pulmonary tuberculosis. Ind L Tub. 1991;38:139.
- Afrasiabian S, Mohsenpour B, Bagheri KH, Sigari N, Aftabi K. Diagnostic value of serum adenosine deaminase level in pulmonary tuberculosis. J Res Med Sci. 2013;18(3):252–4.
- Dilmac A, Ucoluk GO, Ugurman F, Gozu A, Akkalyoncu B, Eryilmaz T. The diagnostic value of adenosine deaminase activity in sputum in pulmonary tuberculosis. Respir Med. 2002;96(8):632–4.
- Salmanzadeh S, Tavakkol H, Bavieh K, Alavi SM. Diagnostic Value of Serum Adenosine Deaminase (ADA) Level for Pulmonary Tuberculosis. Jundishapur J Microbiol. 2015;8(3):e21760.
- Atta S, Kassem A, Elhadidi A, Esawy HE. The diagnostic value of adenosine deaminase activity in pulmonary tuberculosis: Comparison between sputum and serum. Egypt J Chest Dis Tuberc. 2015;64(1):103–7.
- Al-Shammary FJ. Adenosine deaminase activity in serum and pleural effusions of tuberculous and non-tuberculous patients. Biochem Mol Biol Int. 1997;43(4):763–79.
- Conde MB, Marinho SR, Pereira MDF, Silva JRL, Saad MHF, Sales CL, et al. The usefulness of serum adenosine deaminase 2 (ADA2) activity in adults for the diagnosis of pulmonary tuberculosis. Respir Med. 2002;96(8):607–10.
- Badade ZG, Narshetty GS, Shah VK, Potdar PV, More K, Badade VZ. Study of Serum Adenosine Deaminase (ADA) Level in Diagnosis of Extrapulmonary and Smear Negative Tuberculosis. 2015;4(12):4–6.
- Chander A, Shrestha CD. Diagnostic Value of Serum Adenosine Deaminase Levels in Sputum Smear Negative Pulmonary Tuberculosis Patients in Nepalese Population. Asian Pac J Trop Biomed. 2012;2(3):S1896–9.
- Titarenko OT, D’iakova ME, Perova TL, Riasnianskaia TB. The activity of adenosine deaminase and its isoenzymes in patients with different forms of pulmonary tuberculosis. Probl Tuberk. 2002;(3):43– 5.
- Bolursaz MR, Khalilzadeh S, Khodayari A, Hakimi S. Adenosine Deaminase Level as an Indicator for Differentiating Between Active Pulmonary Tuberculosis Infection and Other Pulmonary Infections. J Compr Ped. 2012;3(1):3–6.
- Hassanein K, Hosny H, Mohamed R, Moneim AE. Role of adenosine deaminase (ADA) in the diagnosis of pulmonary tuberculosis. Egypt J Bronchol. 2010;4:11–8.
- Farazi A, Moharamkhani A, Sofian M. Validity of serum adenosine deaminase in diagnosis of tuberculosis. Pan Afr Med J. 2013;15:133.