Blood safety is a significant concern in India due to the high prevalence of infectious agents among donors. According to an Indian study, the prevalence rates of various infections among donors were as follows: Hepatitis B Virus (HBV) at 1.111%, Hepatitis C Virus (HCV) at 0.431%, HIV-I/II at 0.2%, syphilis at 0.29%, and malaria parasite (MP) at 0.006%.1 Minimizing transfusion-transmitted infections (TTIs) is crucial for safe blood transfusions. Enzyme-Linked Immunosorbent Assays (ELISAs) are vital for screening transfusion-transmitted infections (TTIs) such as HIV, Hepatitis B, Hepatitis C, Syphilis and Malaria. They offer high sensitivity and specificity, allowing for the efficient processing of many samples to ensure blood safety. By detecting infected donations, ELISAs help prevent the transmission of serious infections through blood transfusions. Safety measures include donor evaluation, highly sensitive laboratory screening tests, and pathogen inactivation procedures to reduce TTI risk.
Safe transfusion practices are crucial in blood banking due to their significant impact on patient safety and public health. Transfusion safety ensures that the blood or blood products administered to patients are free from infectious agents, compatible with the recipient's blood type, and are of high quality. Failure to adhere to these safety standards can lead to serious health complications, including transfusion-transmitted infections (TTIs), immune reactions, and even death.
- Preventing Transfusion-Transmitted Infections (TTIs): The primary concern in transfusion safety is the risk of TTIs such as HIV, hepatitis B and C, and other pathogens. Rigorous screening and testing protocols are essential to detect and eliminate contaminated blood products.
- Ensuring Compatibility: Blood type mismatches can trigger severe immune reactions, leading to conditions like hemolytic transfusion reactions (HTRs). These reactions can cause symptoms ranging from fever and chills to severe kidney damage and cardiovascular collapse. Thus, compatibility testing, including ABO and Rh typing, is a critical step in transfusion procedures.
- Maintaining Blood Quality: Blood products must be stored and handled correctly to maintain their efficacy and safety. Factors such as temperature control, proper labeling, and adherence to expiration dates are vital in ensuring that patients receive high-quality blood.
- Minimizing Adverse Reactions: Besides infections and compatibility issues, other adverse reactions such as allergic responses, febrile non-hemolytic transfusion reactions (FNHTRs), and transfusion-related acute lung injury (TRALI) can occur. Safe transfusion practices help in identifying and mitigating these risks.
Erba Transasia’s role in contributing to Blood Transfusion Safety
We, at Erba Transasia, are committed to offering advanced diagnostic solutions for blood banks. Our state-of-the-art equipment, including hematology analyzers, immunoassay systems, and coagulation analyzers, ensures precise and accurate testing. Innovative screening technologies detect highly sensitive and specificity infectious agents, reducing the risk of TTIs. Adhering to international standards, Erba Transasia ensures high-quality control and regulatory compliance in blood testing and processing.
Our total diagnostic solutions for screening transfusion transmissible infections (TTIs) confer early and accurate detection of TTIs. The comprehensive product portfolio consists of ELISA screening kits – ErbaLisa HIV Gen 4, ErbaLisa SEN and PICO HBsAg, ErbaLisa HCV Gen4 Ag + Ab, ErbaLisa Syphilis, ErbaLisa Malaria PAN (pLDH), etc. Our ErbaLisa range includes HCV Gen 4 Ag + Ab kits for core antigen and antibody detection, HCV Gen 3 V2 kits for qualitative antibody detection, and HIV Gen 4 kits for simultaneous p24 Antigen and Antibodies to HIV 1 and HIV 2 detection. The ErbaLisa PICO and SEN HBsAg kits employ sandwich ELISA for qualitative hepatitis B virus detection with heightened analytical sensitivity. Additionally, our ErbaLisa Syphilis screening kits allow qualitative determination of total antibodies (IgM, IgA, and IgG) for Treponema pallidum. Designed for blood banks and clinical laboratories, these ELISA kits ensure best-in-class sensitivity, specificity, and accurate results in screening and diagnosis. The ErbaLisa PAN (pLDH) helps in the detection of various malarial parasitic infections.
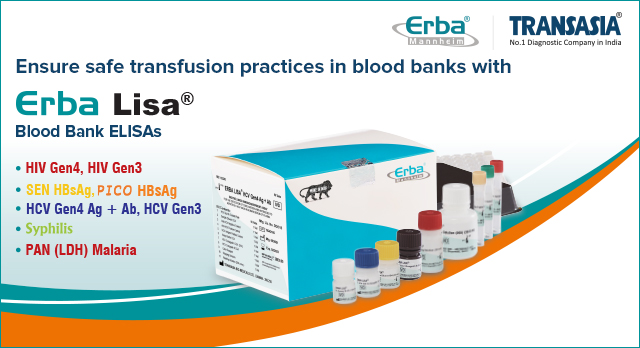
Safe blood transfusion is a cornerstone of effective blood banking, and in-vitro diagnostics plays a critical role in ensuring these practices are upheld. Through our advanced diagnostic tools, screening technologies, and comprehensive support systems, Erba Transasia significantly enhances the safety and reliability of blood transfusions, ultimately safeguarding patient health.
References:
- Thakur SK, Singh S, Negi DK, Sinha AK. Prevalence of TTI among Indian blood donors. Bioinformation. 2023;19(5):582-589. Published 2023 May 31. doi:10.6026/97320630019582